Chemical Kinetics app for iPhone and iPad
Developer: Roman Volinsky
First release : 13 Apr 2015
App size: 2.48 Mb
The “Chemical Kinetics” is an ultimate tool for solving all kind of chemical kinetics problems. It deals with time dependent quantitative analysis of chemical reactions. Specifically, this application solves chemical reaction rate equations and provides graphical representation of reagent and product concentrations vs time. Additionally, app calculates half-life times and equilibrium constants, where it is appropriate.
Theoretical Background:
For a general chemical reaction:
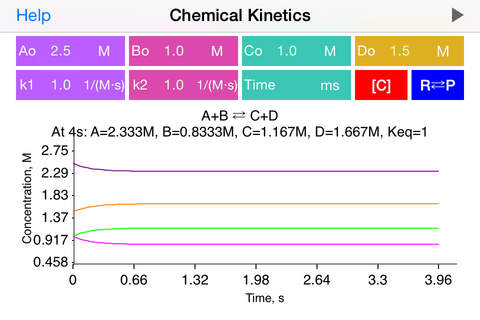
aA + bB => cC + dD,
the reaction rate is defined by:
rate=k * A^x * B^y
where k – is a rate coefficient and sum of powers x and y determines the reaction order. Importantly, actual powers x and y may differ from reaction coefficients a and b, due to overall reaction mechanism complexity.
The application features:
· User is provided with list of basic mono-step unidirectional and equilibrium reactions, where reaction coefficients are actually represent powers and therefore define reaction order:
2A => B, rate = k[A]^2
A+B = C+D, rate = k1[A] [B] - k2[C] [D]
· k1 is a rate coefficient of forward reaction in equilibrium process or rate coefficient for first step in multistep process. Similarly, k2, when available, is a rate coefficient of backward reaction in equilibrium process or rate coefficient for second step in multistep process.
· To start plotting the reaction data initially or after updating the concentration or coefficient fields, user is requested to tap Run button. Run button is greyed out immediately after successful calculation.
· Complete reaction information is provided on top of the plot: concentrations at certain time point (defined by user or provided automatically), optionally equilibrium constant value and half-life time.
· When time field is empty, the app automatically determines the time range. To redefine reaction time limit and find out component concentrations at the altrnative time point, user may update the time field.
· Significant attention should be paid to units of rate coefficients. App automatically changes the set of available units as per order of reaction.
Units acronyms: d - days, h - hours, a - atmosphere, s - seconds, min - minutes, y - years, M -molars, Pa - pascals.
· The basic conversions are as follows:
M-1·s-1 = (1e-3) mM-1·s-1 = (60e-3) mM-1·min-1 = (3600) M-1·h-1
1 atm=101325 Pa